Step-by-step explanation:
According to the conservation of mass, mass can neither be created nor it can be destroyed. But it can only be changed from one form to another.
For example,
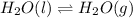
During this change only state of water is changing from liquid to gas and gas to liquid and no change in mass is taking place.
As mass of liquid water = (2 + 16) g/mol = 18 g/mol
Mass of water vapor = (2 + 16) g/mol = 18 g/mol
Hence, this shows that when water vapors are cooled then it changes into liquid state of water without causing any change in mass as mass is conserved.