Answer:- False.
Explanations:- One mole of any gas at STP occupies 1 volume of 22.4 L.
STP means standard temperature and pressure. Standard temperature is 273 K and standard pressure is 1 atm.
Here, in the statement the given temperature is 298 K which is not the standard temperature and so the volume of 1 mole of the gas will not be 22.4 L.
The volume could be calculated using ideal gas equation:
PV = nRT
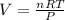
R is the universal gas constant and it's value is
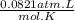 .
.
let's plug in the values and calculate the volume for 1 mole of the gas:
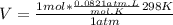
V = 24.5 L
The volume for 1 mol of the gas at 298 K and 1 atm is 24.5 L and hence the statement is false.