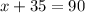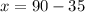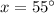Answer:
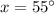

Explanation:
From the question we are told that
Sum of two angle =90
First angle is 15 less than twice the measure of the second angle.
Generally interpreting mathematically we have
Let x be the first angle and second angle y
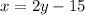
Therefore
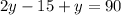
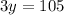
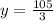

Therefore