Answer: A) Bent or angular, polar
Step-by-step explanation:
The central atom oxygen has two lone pairs and two bond pairs in
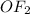 . The number of electron pairs are 4, that means the hybridization will be
. The number of electron pairs are 4, that means the hybridization will be
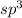 and the electronic geometry of the molecule will be tetrahedral. But as there are two lone pair of atoms around the central oxygen atom, repulsion between lone and bond pair of electrons is more and hence the molecular geometry will be bent shape.
and the electronic geometry of the molecule will be tetrahedral. But as there are two lone pair of atoms around the central oxygen atom, repulsion between lone and bond pair of electrons is more and hence the molecular geometry will be bent shape.
The compound
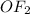 is polar as the net dipole moment of oxygen - fluoride bonds do not cancel each other out.
is polar as the net dipole moment of oxygen - fluoride bonds do not cancel each other out.