Answer: The ion charge formed by Group I will be +1, by Group VI will be -2 and by Group III will be +3.
Step-by-step explanation:
Ions are formed when electrons are lost or gained by an atom. When an atom looses electrons, it forms a positive ion known as cations and when an atom gains electrons, it forms a negative ion known as anions.
The electronic configuration of the elements present in
- Group I:
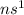 where n is the number of period
where n is the number of period
This configuration will loose 1 electron and thus will form an ion of +1.
- Group VI:
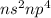 where n is the number of period
where n is the number of period
This configuration will gain 2 electrons and thus will form an ion of -2.
- Group III:
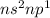 where n is the number of period
where n is the number of period
This configuration will loose 3 electrons and thus will form an ion of +3.
Hence, the ion charge formed by Group I will be +1, by Group VI will be -2 and by Group III will be +3.