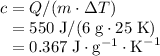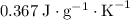
The specific heat of a substance measures the energy required to raise the temperature of a one-unit-mass sample of this substance by one unit.
The question provides the following information:
- Energy change
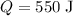
- Temperature change
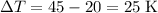
- Mass of the sample
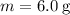
The specific heat of this substance measures the energy change
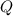 for a process involving:
for a process involving:
- Temperature change
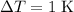
- Mass of the sample
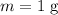
Apply the formula