Given the volume of HCl solution = 30.00 mL
Molarity of HCl solution = 0.1000 M
Molarity, moles and volume are related by the equation:
Molarity =
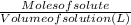
Converting volume of HCl from mL to L:
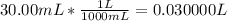
Calculating moles of HCl from volume in L and molarity:
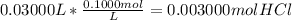
The final moles would be reported to 4 sig figs. So the correct answer will be 0.03000 mol HCl
Correct option: C. 0.03000mol