Answer:-
Solution:- For covalent compounds we consider molecules where as for the ionic compounds we consider the formula units. One mol of a covalent compound equals to Avogadro number of molecules. Similarly, for ionic compounds, one mol equals to Avogadro number of formula units.
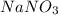 is an ionic compound. So, 1 mol of
is an ionic compound. So, 1 mol of
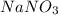 equals to
equals to
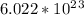 formula units.
formula units.
We are asked to calculate the number of formula units in 9.82 moles of
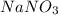 .
.
Given number of moles are multiplied by Avogadro number to get the number of formula units:
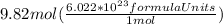
=
 formula units
formula units