Answer: The given statement is false.
Step-by-step explanation:
Voltage in an electrochemical cell helps in determining that the system is out of equilibrium.
When a redox reaction approaches towards equilibrium then there occurs flow of electrons with the advancement in chemical reaction. This means as the chemical reaction proceeds towards completion then flow of electrons also takes place.
Hence, cell potential decreases until the reaction reaches at equilibrium where,
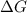 = 0.
= 0.
Also,
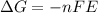 . So, at equilibrium voltage becomes equal to zero and current also stops.
. So, at equilibrium voltage becomes equal to zero and current also stops.
Thus, we can conclude that the statement when equilibrium is reached in an electrochemical cell, the voltage reaches its maximum value is false.