Answer:
The new volume of the air inside when the cabin is pressurized to 75 kPa is 37818.
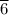 m³
m³
Step-by-step explanation:
The given 747 airplane parameters are;
The volume of air in the airplane, V₁ = 28,000 m³
The pressure of the air on the ground, P₁ = 101.3 kPa
The pressure of the air once it is in flight, P₂ = 75 kPa
Let V₂ represent the new volume of the air inside when the cabin is pressurized to 75 kPa
Boyle's Law states that the pressure of a given mass of air is inversely proportional to its volume at constant temperature
Therefore, by Boyle's law, we have;
P ∝ 1/V and P₁·V₁ = P₂·V₂
Which gives;
V₂ = P₁·V₁/P₂
Substituting the known values, we get;
V₂ = 101.3 kPa × 28,000 m³/(75 kPa) = 37818.
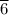 m³
m³
The new volume of the air inside when the cabin is pressurized to 75 kPa = V₂ = 37818.
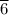 m³.
m³.