Answer: 0.297 moles of calcium carbonate.
Solution:
Number of moles of HCl =
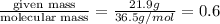 moles
moles
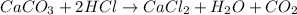
According to reaction
Two moles of HCl are reacting with one mole of
 , then 0.6 moles of HCl will react with:
, then 0.6 moles of HCl will react with:
=
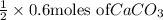
Moles of calcium carbonate reacted = 0.3 moles
In 0.3 moles of calcium carbonate 10% impurities is present i.e.
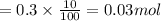 of impurity.
of impurity.
Actual number of moles of calcium carbonate reacted = 0.3 - 0.003 = 0.297 moles.