Answer : The correct answer is the Bonds were broken on the reactants and new bonds were formed on the products.
Explanation :
In the chemical reaction, some substances react together are called reactant and the substance are formed are called product.
During the chemical reaction, the atoms of reactants rearranged to make products. There are on atoms are added or taken away in the reaction. This is known as the conservation of atoms.
For example : carbon atom react with the oxygen to form carbon dioxide.
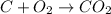
From the given diagram, we conclude that the arrangement of molecules are different on both side of the mixture of reaction.
On the reactant side, the red molecules bonded with red molecule and the black molecule with white molecules. On the other hand i.e product side, the red molecule bonded with black molecule and white molecule bonded with red molecules. The molecular arrangement are different on both side of the reaction mixture.
Therefore, the correct answer is the Bonds were broken on the reactants and new bonds were formed on the products.