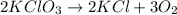Answer : The decomposition reaction will be, (A)
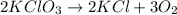
Explanation :
Decomposition reaction : It is defined as the reaction in which a single larger compound decomposes to produce two or more smaller molecules as a product.
The general representation of decomposition reaction is :
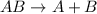
From the given options,
(A)
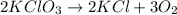 this reaction a decomposition reaction in which a potassium chlorate decomposes to give potassium chloride and oxygen gas as a product.
this reaction a decomposition reaction in which a potassium chlorate decomposes to give potassium chloride and oxygen gas as a product.
While the other reactions,
(B)
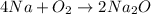 this reaction is a combination reaction in which the two or more molecules combine to form a larger single molecule as a product.
this reaction is a combination reaction in which the two or more molecules combine to form a larger single molecule as a product.
(C)
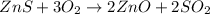 this is a double displacement reaction in which the cation and the anion of the two reactant exchange their places to give two another compounds as a product.
this is a double displacement reaction in which the cation and the anion of the two reactant exchange their places to give two another compounds as a product.
(D)
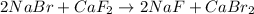 this is a double displacement reaction in which the cation and the anion of the two reactant exchange their places to give two another compounds as a product.
this is a double displacement reaction in which the cation and the anion of the two reactant exchange their places to give two another compounds as a product.
Hence, the decomposition reaction will be,