Answer: The correct option is Metal + polyatomic anion.The name will be 'Calcium carbonate'.
Solution:
Salts name is made up two parts:
- First part is name of cation here present in salt.
- Second part is name of polyatomic anion like :
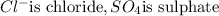 etc.
etc.
So,the name of
 is calcium carbonate.Here,metal ion in the salt is calcium metal and the anion is polyatomic carbonate ion.
is calcium carbonate.Here,metal ion in the salt is calcium metal and the anion is polyatomic carbonate ion.