Isotopes can represented in two ways. In the first way, the element's symbol is used with mass number at the upper left and atomic number written on the lower left of the symbol. In the other form of representation, the isotope name is written with a dash and mass number.
Given that the isotope has 92 protons. So the atomic number is 92.
The isotope has 143 neutrons
So the mass number = Total number of protons + Neutrons
= 92 + 143 = 235
Therefore, the two ways of representing the isotope is:
1.
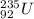
2. Uranium-235