Chemical Equation:
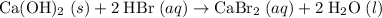
Hence the sum of all four coefficients would be six.
Calcium hydroxide
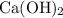 dissolves in water to produce hydroxide ions
dissolves in water to produce hydroxide ions
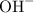 . Calcium hydroxide can thus act as a base.
. Calcium hydroxide can thus act as a base.
Bromine is found in group seventeen of the periodic table and is a halogen element. Molecules of hydrogen bromide
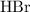 contain hydrogen atoms bonded to bromine atoms. These molecules would thus ionize when dissolved in water to release hydrogen ions
contain hydrogen atoms bonded to bromine atoms. These molecules would thus ionize when dissolved in water to release hydrogen ions
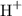 and therefore act as acids.
and therefore act as acids.
Acids would neutralize bases in an aqueous solution. Hydrogen ions would combine with hydroxide ions to produce water.
Calcium hydroxide dissolves to produce calcium ions
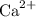 in addition to hydroxide ions. Similarly, hydrogen bromide dissolves to produce bromide ions
in addition to hydroxide ions. Similarly, hydrogen bromide dissolves to produce bromide ions
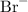 in addition to hydrogen ions. The mixture would contain both calcium ions and bromide ions. It would thus behave as a solution of the salt calcium bromide
in addition to hydrogen ions. The mixture would contain both calcium ions and bromide ions. It would thus behave as a solution of the salt calcium bromide
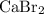 .
.
Thus the reaction would produce two species:
- Calcium bromide
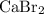 , and
, and - Water
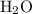
Balance the reaction through the conservation of atoms. For example, start by assuming one as the coefficient of calcium bromide. Both sides of the equation shall thus contain one calcium atom and two bromine atoms. There would be one formula unit of calcium hydroxide and two hydrogen bromide molecules on the reactant side. The formula unit of calcium hydroxide contains two oxygen atoms, which corresponds the production of two water molecules.