ANSWER
The correct answer is A
EXPLANATION
From the equation
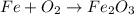
L.H.S.
Fe = 1
O =2
R.H.S
Fe =2
O =3
Now, let try to balance the oxygen atoms first,
To do this, we know the Least Common Multiples of 2 and 3 is 6
To get 6 atoms of oxygen at L.H.S multiply 2 by 3 and on the R.H.S, multiply 3 by 2
L.H.S.
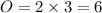
R.H.S
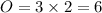
This means that, 3 should be in front of
 at the L.H.S and 2 should come before
at the L.H.S and 2 should come before
 in the R.H.S of the equation
in the R.H.S of the equation
Now, we have
L.H.S.
Fe = 1
O = 6
R.H.S
Fe =4
O =6
The equation is still not balanced since Fe is not balance
The Least Common Multiple of 1 and 4 is 4
Which means that we need to multiply 1 by 4 at the L.H.S and 4 by 1 at the R.H.S
L.H.S.
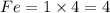
R.H.S
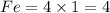
We now, have
L.H.S.
Fe= 4
O=6
R.H.S
Fe =4
O=6
Hence the balanced equation is
