Answer:
3.39 x 10²³ particles
6.02 x 10²²particles
Step-by-step explanation:
The problem here entails we find the number of particles in the given substances.
From the mole concept;
1 mole of a substance contains 6.02 x 10²³ particles
a. 18g of O₂;
We first find the number of moles of this substance first;
Number of moles =
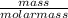
Molar mass of O₂ = 2(16) = 32g/mol
Number of moles =
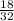 = 0.56mole
= 0.56mole
So;
1 mole of a substance contains 6.02 x 10²³ particles
0.56 mole of O₂ will contain 0.56 x 6.02 x 10²³ particles
= 3.39 x 10²³ particles
b. 0.1mole of calcium atom:
1 mole of a substance contains 6.02 x 10²³ particles
0.1 mole of the substance will contain 0.1 x 6.02 x 10²³ particles
= 6.02 x 10²²particles