Answer: Option (B) is the correct answer.
Step-by-step explanation:
Lithium is an alkali metal with atomic number 3 and its electronic distribution is 2, 1.
So, in order to attain stability it needs to lose its one valence electron and thus it becomes
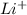 ion.
ion.
Hence, an ionic bond will always be formed when a metal reacts with a non-metal. As atomic number of fluorine is 9 and its electronic configuration is 2, 7.
So, fluorine on gaining one electron from lithium will form LiF compound.
Whereas neon is a noble gas so it will be unreactive in nature and silver and calcium being metals will not be able to combine with lithium which is also a metal.
Thus, we can conclude that fluorine (F) is the element which is likely to combine with lithium.