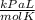The formula for the Ideal Gas Law is: PV = nRT
The ideal gas constant is R, and by simply rearranging the formula, the units can be determined.
R =
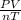
The units for Pressure is kPa, Volume is L, n, or mole number is mol, Temperature is K.
Thus the units for R are: