Dipole-dipole interactions.
There are three classes of intermolecular interactions: London dispersion force, Dipole-dipole interaction, and hydrogen bonds.
Hydrogen bonds are observed only in molecules with hydrogen atoms bonded directly to atoms of fluorine, oxygen, or nitrogen that are of high electronegativity. Chloromethane
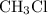 contains no such bonds. Therefore, there would be no hydrogen bonds between molecules of
contains no such bonds. Therefore, there would be no hydrogen bonds between molecules of
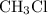 .
.
Dipole-dipole interactions are weaker than hydrogen bonds in most molecules. Dipole-dipole interactions exist only between polar molecules. Chloromethane contains polar carbon-chlorine bonds. Chloromethane molecules are asymmetric, such that polarity due to the presence carbon-chlorine bonds does not cancel out within the molecule. Chloromethane molecules are therefore polar, and would experience dipole-dipole interactions.
London distribution forces attribute to the movement of electrons within a molecule. Such interactions are transient and thus weaker than both hydrogen bonds and dipole-dipole interactions in small molecules such as
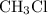 .
.