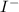Answer: To achieve noble gas configuration and become stable.
Step-by-step explanation:
Ions are formed when an atom looses or gains electrons.
If an atom gains electrons, it leads to the formation of negative ions known as anions. If an atom looses electrons, it leads to the formation of positive ions known as cations.
As Iodine has atomic number of 53, it contains 53 electrons which are filled according to Afbau's rule as:
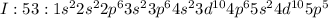
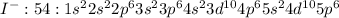
As iodine has 7 valence electrons it is valence shell 5 , it is short of one electron to achieve nearest noble gas configuration of Krypton with atomic number of 54. The elements which follow octet rule are considered to be stable and thus iodine accepts electron to form