Answer:
2.94 x 10⁻¹⁴L
Step-by-step explanation:
To solve this problem, we have to assume that the condition of this water is at standard temperature and pressure, STP.
At STP;
1 mole of gas have a volume of 22.4L
So, let us find the number of moles of this water first;
6.02 x 10²³ molecules can be found in 1 mole of a substance
7.89 x 10⁸ molecules will contain
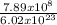 = 1.31 x 10⁻¹⁵mole of water
= 1.31 x 10⁻¹⁵mole of water
So;
Volume of water = 22.4 x 1.31 x 10⁻¹⁵ = 2.94 x 10⁻¹⁴L