Approximately
 .
.
The relative atomic weight of an element is a weighted average of the atomic mass for all its isotopes. It accounts for the atomic mass for each isotope, as well the relative abundance of each.
The question provides no detailed data on the relative mass for each isotope atom. Each nucleon- proton or neutron- has a mass of approximately
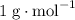 . The mass number of an atom gives the total number of nucleons it contains. The mass number can, therefore, serve as a numerical estimator for the relative atomic mass of an isotope. That is:
. The mass number of an atom gives the total number of nucleons it contains. The mass number can, therefore, serve as a numerical estimator for the relative atomic mass of an isotope. That is:
- Copper-63 has mass number 63 and a relative atomic mass of approximately
 ;
; - Copper-65 has mass number 65 and a relative atomic mass of approximately
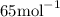
Copper-63 has an abundance of 69.17% relative to all copper atoms. Similarly, copper-65 has a relative abundance of 30.83%. Therefore, one would expect to find 6,917 copper-63 atoms and 3,083 copper-65 atoms in a sample of 10,000 copper atoms. Similarly, 10,000 moles or 10,000 × (6.02 × 10²³) copper atoms would contain 6,917 moles of copper-63 atoms and 3,083 moles of copper-65 atoms.
10,000 moles of copper atoms would have a mass of approximately
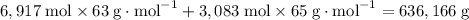 .
.
Each mole of copper atoms would thus have a mass of approximately
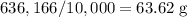 .
.
Combining the two previous steps would give:
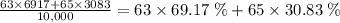 ,
,
which essentially multiplies the relative atomic mass by their relative abundance, and takes the sum of the products.
The relative atomic mass of an element is measured in grams per mole. One mole of copper atoms have a mass of 63.62 grams. Copper thus has a relative atomic mass of
 .
.