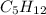Answer:
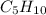 has empirical formula of
has empirical formula of
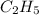
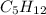 has empirical formula of
has empirical formula of
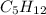
Step-by-step explanation:
Molecular formula is the chemical formula which depicts the actual number of atoms of each element present in the compound.
Empirical formula is the simplest chemical formula which depicts the whole number of atoms of each element present in the compound.
To find empirical formula, the molecular formula is divided to convert them into simplest whole number ratios.
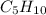 has empirical formula of
has empirical formula of
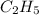
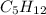 has empirical formula of
has empirical formula of