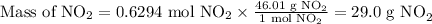Answer:
29.0 g
Step-by-step explanation:
We know that we will need a chemical equation with masses and molar masses, so let’s start by gathering all the information in one place.
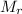 : 63.55 46.01
: 63.55 46.01
Cu + 4HNO₃ ⟶ Cu(NO₃)₂ + 2NO₂ + 2H₂O
Mass/g: 20.0
1. Use the molar mass of Cu to calculate the moles of Cu.
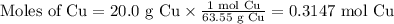
2. Use the molar ratio of NO₂:Cu to calculate the moles of NO₂.
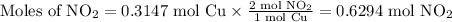
3. Use the molar mass of NO₂ to calculate the mass of NO2.