Answer: The correct answer is Option B.
Step-by-step explanation:
Oxidizing agent is the agent which oxidizes the other substance and itself gets reduced. It undergoes reduction reaction in which, it gains electrons and also the oxidation state of the substance gets reduced.
Reducing agent is the agent which reduces the other substance and itself gets oxidized. It undergoes oxidation reaction in which, it looses electrons and the oxidation state of the substance is increased.
For the given chemical equation:
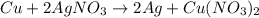
Half reactions for the given chemical reaction:
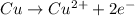
Copper is loosing 2 electrons, thus it is undergoing oxidation reaction and is considered as a reducing agent.
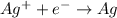
Silver ion is gaining 1 electron per atom, thus it is undergoing reduction reaction and is considered as an oxidizing agent.
Hence,
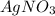 is considered as an oxidizing agent and therefore the correct answer is Option B.
is considered as an oxidizing agent and therefore the correct answer is Option B.