Answer: A. NO increases the rate at which
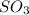 molecules are formed.
molecules are formed.
Explanation: The catalyst makes it possible for the reaction to take place by another path that makes possible reaction at a lower energy.
Activation energy is the extra energy that must be supplied to reactants in order to cross the energy barrier and thus convert to products.
A catalyst is a substance which increases the rate of a reaction by taking the reaction through a different path which involves lower activation energy and thus more reactants
 and
and
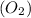 undergo collision and can cross the energy barrier and convert to products
undergo collision and can cross the energy barrier and convert to products
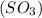
Thus NO increases the rate at which
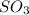 molecules are formed.
molecules are formed.