Answer: The average atomic mass of the given element is 15.99438 u
Step-by-step explanation:
Isotope-A atomic mass = 15.99 u
Isotope-A fractional abundance = 99.762 % = 0.99762
Isotope-B atomic mass = 16.99 u
Isotope-B fractional abundance = 0.038 % = 0.00038
Isotope-C atomic mass = 17.99 u
Isotope-C fractional abundance = 0.200 % = 0.00200
Average atomic mass of an element :
=
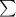 (Atomic mass of an isotope) × (respective percent abundance)
(Atomic mass of an isotope) × (respective percent abundance)
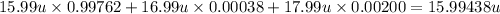
The average atomic mass of the given element is 15.99438 u