Answer: The correct answer is Option C.
Step-by-step explanation:
Oxidation reaction are the reactions in which an element or compound looses electrons and the element that looses electrons is known as oxidized species. The oxidation state of the species is increased for these kind of reactions.
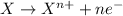
Reduction reaction are the reactions in which an element or compound gains electrons and the element that gains electrons is known as reduced species. The oxidation state of the species gets reduced for these kind of reactions.
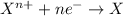
For the given chemical reaction:
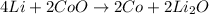
On reactant side:
Oxidation state of lithium = 0
Oxidation state of cobalt = +2
Oxidation state of oxygen = -2
On product side:
Oxidation state of lithium = +1
Oxidation state of cobalt = 0
Oxidation state of oxygen = -2
As, oxidation state of lithium is increasing, it is loosing electrons and thus it is undergoing oxidation reaction and the oxidation state of cobalt in reducing, it is gaining electrons and thus it is undergoing reduction reaction .
Hence, the correct answer is Option C.