Answer: The correct answer is Option A.
Step-by-step explanation:
A catalyst is defined as the substance which increases the rate of the reaction without actually participating in the reaction.
This substance decreases the activation energy of the reactants, so as to proceed the reaction at a faster rate by increasing their effective collisions.
It does not affect the concentration of the reactants.
For the given reaction:
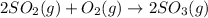
If we add NO, which is acting as a catalyst will increase the rate of the reaction to produce the products which is
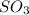
Hence, the correct answer is Option A.