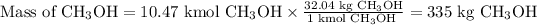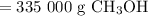Answer:
335 000 g
Step-by-step explanation:
We know that we will need a chemical equation with masses and molar masses, so let’s start by gathering all the information in one place.
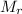 : 2.016 32.04
: 2.016 32.04
CO + 2H₂ ⟶ CH₃OH
Mass/kg: 42.2
1. Use the molar mass of H₂ to calculate the moles of H₂.
Note: 1 g/mol ≡ 1 kg/kmol
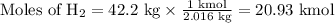
2. Use the molar ratio of CH₃OH:H₂ to calculate the moles of CH₃OH.
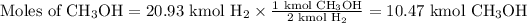
3.Use the molar mass of CH₃OH to calculate the mass of CH₃OH.