Answer : The correct option is, (C)

Solution for Part 1 : Given,
Mass of
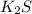 = 235 g
= 235 g
Molar mass of
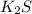 = 110.262 g/mole
= 110.262 g/mole
First we have to calculate the moles of
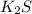 .
.
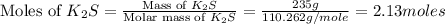
Now we have to calculate the number of atoms of potassium in the given compound.
In the given compound, there are 2 atoms of K and 1 atom of S.
As, 1 mole contains
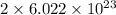 number of potassium atoms
number of potassium atoms
So, 2.13 moles contains
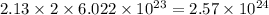 number of potassium atoms
number of potassium atoms
Therefore, the number of potassium atoms in given compound is,

Explanation for Part 2 :
Statement 1 : A balloon shrinks when it's taken outside in the winter.
This statement follow the Charles's law. There is a direct relation between the temperature and volume. As the temperature decreases, the volume also decreases and vice-versa.
Statement 2 : A closed, flexible container expands when it's heated.
This statement follow the Charles's law. There is a direct relation between the temperature and volume. As the temperature increases, the volume also increases and vice-versa.
Statement 3 : When the size of an air chamber is increased, the air pressure decreases.
This statement follow the Boyle's law. There is an inverse relation between the pressure and volume. As the pressure decreases, the volume increases and vice-versa.
Statement 4 : Pressing on an inflated balloon decreases its size.
This statement follow the Boyle's law. There is an inverse relation between the pressure and volume. As the pressure increases, the volume decreases and vice-versa.
Statement 5 : A balloon expands when air is blown into it.
This statement follow the Avogadro's law. There is a direct relation between the number of moles of gas and volume. As the number of moles of air increases, the volume also increases and vice-versa.