Answer:- pH sis 13.63
Solution:- It is a strong base vs strong acid titration. The equation for the reaction takes place between given acid and base is:
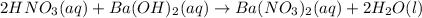
let's calculate the moles of each from given molarities and mL.
moles of barium hydroxide =
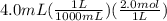
= 0.008 mol
moles of nitric acid =
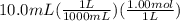
= 0.01 mol
From balanced equation base and acid react in 1:2 mol ratio. So, let's calculate the moles of base used to react with the acid:
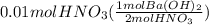
=

excess moles of barium hydroxide = 0.008 - 0.005 = 0.003 mol
Total volume of the solution = 0.004L + 0.010 mL = 0.014 L
Concentration of excess barium hydroxide =
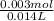
= 0.214M
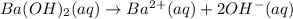
Barium hydroxide as two OH in it. So, the concentration of hydroxide ions will be twice of barium hydroxide concentration.
So,
![[OH^-]=2*0.214M](https://img.qammunity.org/2019/formulas/chemistry/college/6jxbdz547i8i6y19004uyuip8648c4d7v2.png) = 0.428M
= 0.428M
![pOH=-log[OH^-]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/bb6nd3dwmelw6hrf97fgv6xj1ptgdgg61f.png)
pOH = log(0.428)
pOH = 0.37
pH = 14 - pOH
pH = 14 - 0.37
pH = 13.63
First choice is correct, the pH of the solution is 13.63.