Answer:
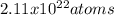
Step-by-step explanation:
Hello!
In this case, considering the Avogadro's number, which relates the number of particles and one mole, we can infer that 1 mole of any element contains 6.022x10²³ atoms as shown below:
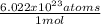
Thus, we compute the number of atoms in 0.035 moles as shown below:
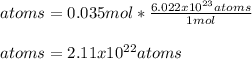
Best regards!