Answer: Charge on the nucleus of an Uut element is +113
Explanation: A nucleus of an element consists of 2 subatomic particles: Protons and neutrons
Charge on 1 neutron = 0
Charge on 1 proton = +1
Number of protons = Atomic Number of the element
Atomic Number of the element Uut = 113
Number of protons = 113
Number of neutrons = Atomic mass - Number of protons
Atomic mass of Uut = 286 amu
Number of neutrons = 286 - 113 = 173
Charge on the nucleus is calculated by:
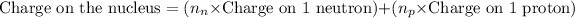
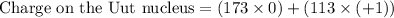
Charge on the Uut nucleus = +113