Answer:- B)
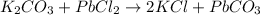
Explanations:- As per the solubility rules, magnesium chloride is soluble. All compounds of alkali metals are soluble, so KCl is soluble. All carbonates are insoluble except the carbonates of alkali metals and ammonium ion.
So,
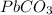 is insoluble and it's precipitate forms.
is insoluble and it's precipitate forms.
LiBr is soluble and all acetates are soluble, so
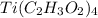 is soluble. All nitrates are soluble and so
is soluble. All nitrates are soluble and so
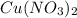 is also soluble.
is also soluble.
Hence, the precipitate forms only in reaction B.