Answer: Option (B) is the correct answer.
Step-by-step explanation:
A chemical reaction in which there is transfer of electrons between any two species is known as a redox reaction.
For example,
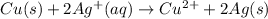
Oxidation-half reaction:
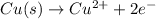
Reduction-half reaction:
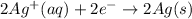
Thus, we can conclude that balancing a redox equation by the half-reaction method is based on action of transfer of electrons.