Atoms are most stable with an a complete valence shell. A complete valence shell typically contain eight electrons for most atoms. Such stable configurations are thus known as "octets." Atoms undergo chemical reactions to achieve octets and, hence, stability.
- An atom might lose or gain one electron or electrons to achieve an octet;
- An atom might share electrons with some other atoms to attain a complete valence shell. It can do so with one or more atoms.
An atom that lose or gain electrons without connecting itself to another atom is known to be ionized. It has either more or less electrons in its electron cloud than protons is has in its nucleus. The atom thus carry a charge. Each electron carries a negative charge whereas each proton carries a positive charge. The charge on electrons and protons cancel out when present in equal numbers. Atoms are thus neutral themselves. An atom that has gained one or more extra electrons becomes a negative ion. An atom that has lost one or more electrons becomes a positive ion.
Ions of opposite charges attract each other to produce ionic compounds. Ionic compounds are typically stable. For instance, table salt
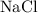 is in large chemically inert.
is in large chemically inert.
Atoms share electrons to achieve octets become connected to each other. For instance, an oxygen atom is two electrons away from attaining a complete valence shell. It might share two electrons with another oxygen atom that also lacks two electrons. Both atoms would thus end up with complete octets. The two atoms are mutually attracted to two shared pairs of electron. They are thus connected to each other. The oxygen molecule
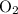 produced in this process is more stable than two separate oxygen atoms with incomplete valence shells. Electron pairs shared are known as bonding pairs of the covalent bond.
produced in this process is more stable than two separate oxygen atoms with incomplete valence shells. Electron pairs shared are known as bonding pairs of the covalent bond.