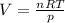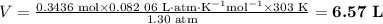Answer:
6.57 L
Step-by-step explanation:
First, calculate the moles of hydrogen produced, then use the Ideal Gas Law to calculate the volume of hydrogen.
Step 1. Write the chemical equation.
 : 22.99
: 22.99
2Na + H₂O ⟶ 2NaOH + H₂
Step 1. Convert grams of Na to moles of Na
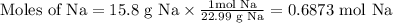
Step 2. Use the molar ratio of H₂:Na to convert moles of Na to moles of H₂.
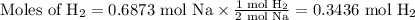
Step 3. Use the Ideal Gas Law to calculate the volume of hydrogen.
pV = nRT