Answer: There are
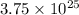 number of H atoms in 7.80 mol of ammonium sulfide.
number of H atoms in 7.80 mol of ammonium sulfide.
Step-by-step explanation:
The formula of ammonium sulfide is
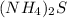 .
.
There are 2 nitrogen atoms, 8 hydrogen atoms and 1 sulfur atom in one molecule of ammonium sulfide.
Calculate moles of hydrogen atoms as follows.
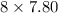 = 62.4
= 62.4
According to Avogadro's number 1 mole contains
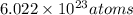
Therefore, calculate number of H atoms as follows.
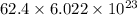
=
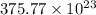
=
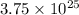
Thus, there are
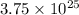 number of H atoms in 7.80 mol of ammonium sulfide.
number of H atoms in 7.80 mol of ammonium sulfide.