Answer:
Mass of
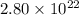 molecules of NaOH = 1.86 g
molecules of NaOH = 1.86 g
Step-by-step explanation:
Avogadro’s number represent the number of the constituent particles which are present in one mole of the substance. It is named after scientist Amedeo Avogadro and is denoted by
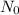 .
.
Avogadro constant:-
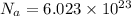
So,
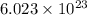 molecules of NaOH are present in 1 mole of NaOH
molecules of NaOH are present in 1 mole of NaOH
Given, Molar mass of NaOH = 40.0 g/mol
This means, mass of 1 mole of NaOH = 40 g
So,
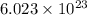 molecules of NaOH weighs 40 g
molecules of NaOH weighs 40 g
1 molecule of NaOH weighs
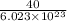 g
g
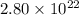 molecules of NaOH weighs
molecules of NaOH weighs
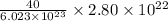 g
g
Mass of
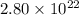 molecules of NaOH = 1.86 g
molecules of NaOH = 1.86 g