Answer: D. mass number
Step-by-step explanation:
An atom is smallest unit of a matter or substance.It consist of three subatomic particles : electrons, protons, neutrons.
Mass number = Number of protons + Number of neutrons
Atomic number=Number of protons =number of electrons (for neutral atom)
Charge is developed on a electrically neutral atom by loss or gain of electrons.
Isotopes are elements which have same atomic number but different mass number. Example:
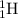 and
and
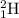 are isotopes of hydrogen with mass number of 1 and 2 respectively.
are isotopes of hydrogen with mass number of 1 and 2 respectively.