Answer:
b) Ethyl alcohol
Step-by-step explanation:
Let’s calculate the amount of heat required to boil away each liquid.
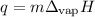
Ethyl alcohol:
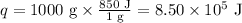
Water:

If we are supplying heat at a constant rate, we will have provided enough to evaporate all the ethyl alcohol while the water is still boiling.
The ethyl alcohol will boil away first.
Evaporation and condensation occur at the same rate only in a closed system .