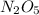Answer : The correct formula of the compound is,
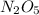
Explanation :
Dinitrogen pentoxide is a covalent compound in which both the elements nitrogen and oxygen are non-metals.
The formula of covalent compound is given by:
- The less electronegative element is written first with prefix number like 'mono' for 1 atom, 'di' for 2 atoms, 'tri' for 3 atoms and so on.... in the subscript.
- The more electronegative element is written then with their prefix number in the subscript.
So, the formula of given compound is,