Answer: (a)The molar mass of the metal is 121.66 g/mol.
(b) The metal hydroxide is
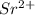 .
.
Step-by-step explanation:
The equation for the reaction is as follows.

(a) When 56.9 mL of the acid is required to reach end point of titration. Then,
Moles of acid =
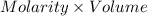
=
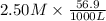
= 0.14225 moles
Hence, the number of moles of hydroxide = 1 - 0.14225 = 0.0711 moles
It is given that mass of sample is 8.65 g, then calculate the molar mass of the sample as follows.
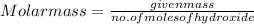
=
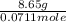
= 121.66 g/mol
The molar mass of the metal is 121.66 g/mol.
(b) The molar mass calculated is 121.66 g/mol. Therefore, calculate the molar mass of each given metal hydroxide as follows.
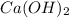 = (40.078 + 34) g/mol = 74.093 g/mol
= (40.078 + 34) g/mol = 74.093 g/mol
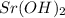 = (87.66 + 34) g/mol = 121.66 g/mol
= (87.66 + 34) g/mol = 121.66 g/mol
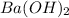 = (137.32 + 34) g/mol = 171.32 g/mol
= (137.32 + 34) g/mol = 171.32 g/mol
Thus, it can be concluded that the metal in the metal hydroxide is
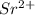 .
.