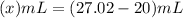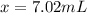Answer: 7.02 mL of water was added in the solution.
Explanation: There is a direct relation of absorbance and concentration which is given by Beer-Lambert's Law.
Mathematically, this law is written as:
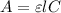
where, A = absorbance
 = Molar extinction coefficient
= Molar extinction coefficient
l = Path length
C = concentration (in terms Molarity)
We are given a copper solution whose absorbance is decreased by 26%.
Assuming that the absorbance of original solution was 100%.
New absorbance will be (100-26)% = 74%
For Original absorbance,
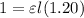 ....(1)
....(1)
For new absorbance,
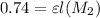 ....(2)
....(2)
As Molar extinction coefficient and path length would be same for both the solutions, dividing equation 2 by 1, we get
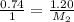

Now, to calculate the volume needed to reduce the abosrbance by 26%, we use the relation:
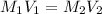
Where,
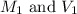 are the molarity and volume of the original solution.
are the molarity and volume of the original solution.
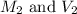 are the molarity and volume of the diluted solution.
are the molarity and volume of the diluted solution.
Putting the values, we get
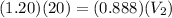
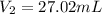
This is the volume of the diluted solution.
To make the original volume of 20 ml to 27.02 ml, water need to be added ,