Answer:
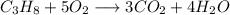
Step-by-step explanation:
The chemical equation for this reaction is without balanced is:
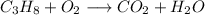
We take oxygen in reactants because these reactions are done in air not in vacuum.
Now , according to law of conservation of matter in any closed system the amount of matter remains constant after transition of matter.
So we need to balance each and every elements present in the above equation i.e. O(oxygen), H(hydrogen) and C(carbon).
So we will follow the standard method of balancing the equation.
First we balance carbon both sides. The carbon in right side should be 3 to balanced with carbon in left side.
Therefore,
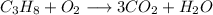
Similarly, we also balance hydrogen .To do so we need 8 hydrogen molecules in right side so we prefix 4 in
 molecule since water itself have two hydrogen molecule they multiply to get total of 8 hydogen atom.
molecule since water itself have two hydrogen molecule they multiply to get total of 8 hydogen atom.
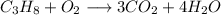
So, till now we have balanced carbon and hydron.
Now, their are total 10 oxygen atom in right hand side. 6 form
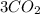
and 4 form
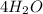 .
.
So , we also balance these and multiplying 5 with
 to get 10 oxygen atoms both sides.
to get 10 oxygen atoms both sides.
And final balanced equation we get is:
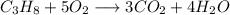
Hence , this is the required solution.