Answer : The correct option is, (3) molecules under the right end of the curve
Explanation :
Evaporation : It is type of process in which the phase changes from liquid state to gaseous state below the boiling temperature of the liquid.
As we know that the higher the temperature of the liquid
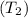 , the more will be the molecule escaping into the gaseous phase than the lower temperature
, the more will be the molecule escaping into the gaseous phase than the lower temperature
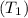 .
.
So, the molecules under the right end of the curve are likely candidates for evaporation.
Hence, the correct option is, (3)