Answer:- mass of water is 0.15 g.
Solution:- This problem is based on the formula:
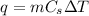
where, q is the heat energy, m is mass,
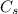 is specific heat and
is specific heat and
 is change in temperature.
is change in temperature.
 = final temperature - initial temperature
= final temperature - initial temperature
 = 37.0 - 5.0 = 32.0 degree C
= 37.0 - 5.0 = 32.0 degree C
m = ?
q = 20.0 J
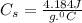
Let's plug in the values in the formula:
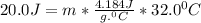
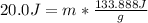
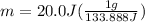
m = 0.15 g
So, the mass of water for the given problem is 0.15 g.